Description
What is it and why does it matter to me?
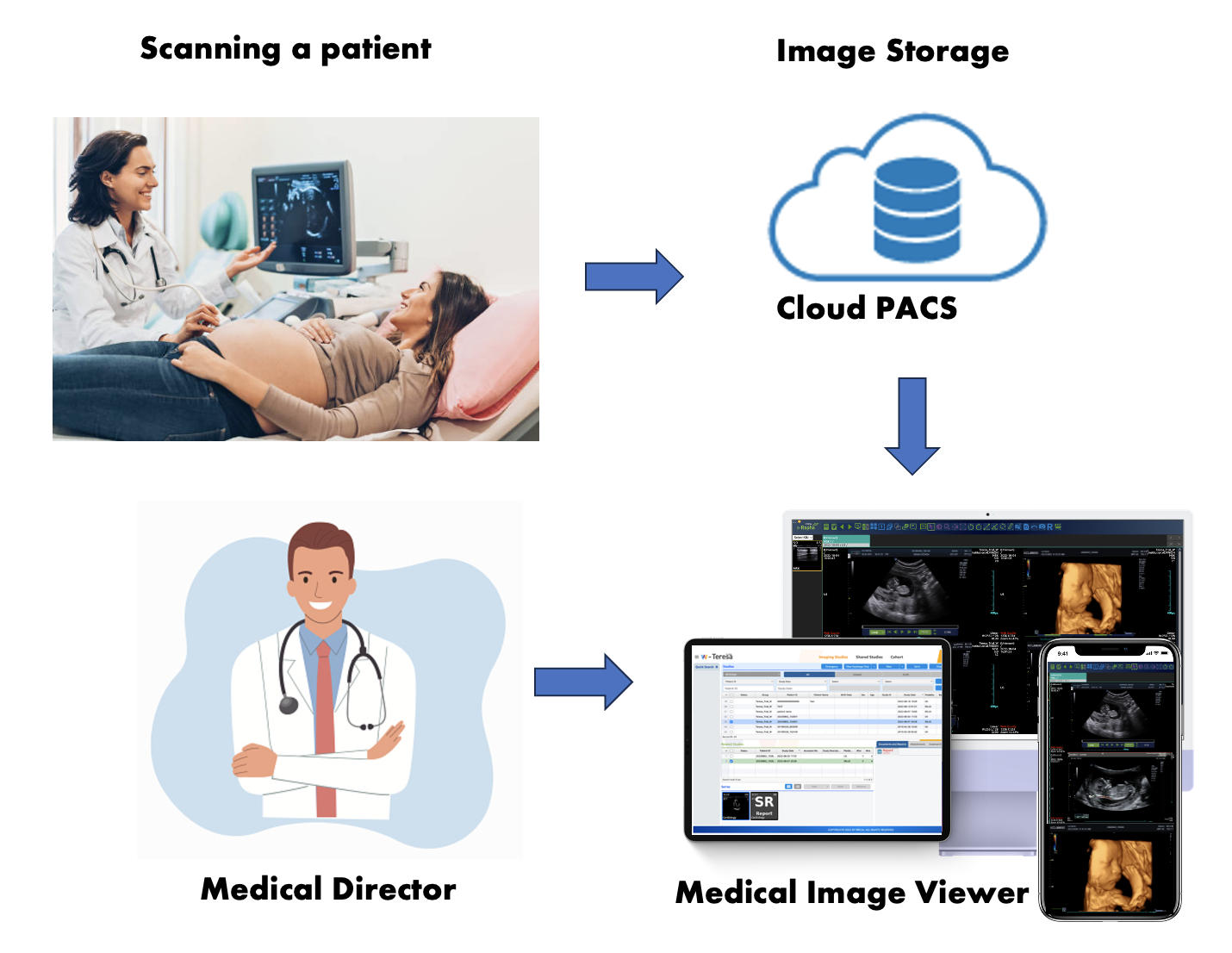
How does my clinic comply with FDA regulations regarding ultrasound image management?
– What does the FDA regulate regarding the management of ultrasound images?
– What challenges do pregnancy centers face in adhering to government regulations?
– How will compliance be planned, and what will the costs be?
Speaker: Julian Lee, Chief Product Officer for Teresa Cloud
Bio: With over two decades in the ultrasound imaging industry, Julian Lee has worn many hats, beginning as an R&D engineer and advancing to strategic roles in global marketing and sales. For 15 years, Julian has honed his expertise in the US market, specializing in ultrasound machines, image management, and comprehensive knowledge of FDA and HIPAA regulations. Holding an MBA from the University of California at Berkeley, Julian Lee is primed to share invaluable insights into navigating FDA compliance in ultrasound image management.
Webinar length:
Between 35-45 minutes with time for Q&A
Reviews
There are no reviews yet.